Make a difference, while working differently


What you can expect to find at BluePath Solutions
-

Innovative problems that stretch you
While we pride ourselves on being trusted with some of the most challenging problems in the industry, we don’t leave you alone to fend for yourself. As you solve them, you’ll receive extensive mentorship and training to refine your skillset for long-term success.
-

Respect for your individuality
Each employee is a different person with unique working styles. Maintaining this individuality is important to us, so we work in a flexible manner to accommodate varied needs. The resultant dynamic relies on self-accountability rather than micromanagement.
-

Opportunities to feel like a core part of the team
At the end of the day, we know we’re here to work. But, we really really like who we do the work with. Expect to be invited regularly to activities outside of the office. Working at BluePath means being an integral part of a skilled, ambitious, close-knit team.

Inclusivity at BluePath
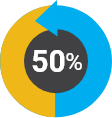 Of women in the workforce
Of women in the workforce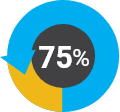 Of our staff have diverse backgrounds
Of our staff have diverse backgrounds
The people at BluePath fit into three broad categories: researchers, clinicians, and marketers. Within these categories, they cover an array of skills needed in the industry. We have health service researchers, epidemiologists, econometricians, doctors, writers, and graphic designers to name a few. What uniqueness will you bring to the table?

The best and brightest at BluePath




